Metals and Non-Metals online mcq quiz
Metals and Non-Metals boostup points
➥ Physical Properties of Metals:
🢂Hardness: Most of the metals are hard, except alkali metals, such as sodium, potassium, lithium, etc. Sodium, potassium, lithium etc. are very soft metals, these can be cut using knife.
🢂 Strength: Most of the metals are strong and have high tensile strength. Because of this big structures are made using metals, such as copper and iron.
🢂 State: Metals are solid at room temperature except mercury.
🢂 Sound: Metals produce ringing sound, so, metals are called sonorous. Sound of metals is also known as metallic sound. This is the cause that
metal wires are used in making musical instruments.
🢂 Conduction: Metals are good conductor of heat and electricity. This is the cause that electric wires are made of metals like copper and aluminium.
🢂 Malleability: Metals are malleable. This means metals can be beaten into thin sheet. Because of this property iron is used in making big ships.
🢂 Ductility: Metals are ductile. This means metals can be drawn into thin wire. Because of this property wires are made of metals.
🢂 Melting and boiling point: Metals have generally high melting and boiling points.
🢂 Density: Most of the metals have high density.
🢂 Color: Most of the metals are grey in color. But gold and copper are exceptions
➥ Physical Properties of nonmetals:
🢂 Physical State: Most of the non-metals exist in two of the three states of matter at room temperature: gases (oxygen) and solids (carbon).
🢂 Non-Malleable and Ductile: Non-metals are very brittle, and cannot be rolled into wires or pounded into sheets.
🢂 Conduction: They are poor conductors of heat and electricity.
🢂Luster: These have no metallic luster and do not reflect light
🢂 Conductivity: Poor conductors of heat and electricity
🢂 Melting and Boiling Points: The melting points of non-metals are generally lower than metals.
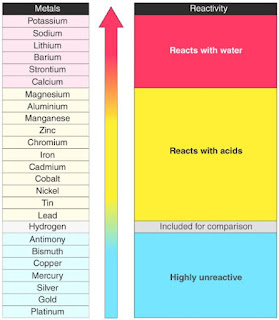
➥ Chemical Properties of Metals:
🢂 Metals are electropositive elements that generally form basic or amphoteric oxides with oxygen.
🢂 Electropositive Character: Metals tend to have low ionization energies, and typically lose electrons (i.e. are oxidized) when they undergo chemical reactions.
🢂 They normally do not accept electrons.
🢂 Most metal oxides are basic oxides and dissolve in water to form metal hydroxides:
When more reactive metal react with solution of less reactive metal salt, more reactive metal displace less reactive metal from its salt.
M1 + M2B(aq) → M1B(aq) + M2
General Reactions:
🢂 Metal oxide + water -> metal hydroxide
🢂 Metal oxide + acid -> salt + water
➥ Chemical Properties of Nonmetals:
🢂 Non-metals have a tendency to gain or share electrons with other atoms.
🢂They are electronegative in character.
🢂Nonmetals, when reacting with metals, tend to gain electrons (typically attaining noble gas electron configuration) and become anions.
General Reactions:
🢂 Nonmetal + Metal -> Salt
🢂 Nonmetal oxide + water -> acid
🢂 Nonmetal oxide + base -> salt + water
➥ Metalloids
🢂 Metalloids have their properties intermediate between the metals and nonmetals.
🢂 Metalloids are useful in the semiconductor industry.
🢂 Metalloids are all solid at room temperature. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi-conductors.
➥ Extraction of Metals:
🢂 An ore is any naturally-occurring source of a metal that you can economically extract the metal from.
🢂 Concentrating the ore: This simply means getting rid of as much of the unwanted rocky material as possible before the ore is converted into the metal.
🢂 In some cases this is done chemically.
🢂 For example, pure aluminium oxide is obtained from bauxite by a process involving a reaction with sodium hydroxide solution. But, in many cases, it is possible to separate the metal compound from unwanted rocky material by physical means. A common example of this involves froth flotation.
🢂 Converting Concentrated ore to Oxide:
🢂 If the concentrated ore is not an oxide of the metal then the ore is first converted into the respective metal oxide.
🢂 This is done by two methods:
🢂 Roasting: Heating the ore in presence of air to convert it into respective metal oxide. Roasting is generally done for sulfide ores.
🢂 Calcination: Heating the ore in limited supply of air to convert it into respective metal oxide. Calcination is generally done for carbonate ores.
🢂 Reduction of metal oxide to metal: The metal oxide is then converted into the metal by reducing the oxide. Depending on the reactivity of the metal, different methods can be used:
🢂Highly reactive metals like Na and Al are reduced by electrolysis.
🢂 Moderately reactive metals like Zn and Fe are reduced using a suitable reducing agent like carbon.
🢂 Metals that have low reactivity are reduced by thermal reduction.
🢂 Refining of metals: The metals thus obtained are purified by different methods such as electrolysis, distillation, etc., depending on the usage and application of the metal.
🢂Corrosion is a natural process, which converts a refined metal to a more chemicallystable form, such as its oxide, hydroxide, or sulfide.
🢂 It is the gradual destruction of materials (usually metals) by chemical and/or
electrochemical reaction with their environment.
Prevention of Corrosion
🢂 Galvanization
🢂 Alloying
🢂 Painting
🢂 Electroplating
Metals and Non-Metals online mcq quiz started from here
Tags : online mcq test for class 10 science
online mcq test for class 10 Metals and Non-Metals
online mcq test for class 10 science chapter 3
online test series for class 10
online test of science class 10 chapter 3
10th online test 2021
10th class online test science
online test for class 10 cbse board
10th class online test 2021
online test for cbse class 10 science
online mcq test for class 10 science
free online test series for class 10 science

0 Comments